Calculate Which Which of the Following Is a Planar Molecule
Hydrazine N2H4 is a colorless liquid used in rocket fuel. H X 2 C C C C H X 2.

Molecular Geometry Boundless Chemistry
The compound must be cyclic.

. Explore more such questions and answers at BYJUS. Sp 3 hybridized state. A Charge of anion.
A-XeF4 B-NF3 C-SiF4 D-SF4 MORE THAN ONE CHOICE. VValence electrons of central atom. The compound must follow Hückels Rule the ring has to contain 4n2 p-orbital electrons.
According to VSEPR what is the structural formula for formamide satisfy this requirement. Memorize flashcards and build a practice test to quiz yourself before your exam. A plane is the surface that two coordinate axes form from algebra.
1 Ethylene C2H4 is a planar molecule but hydrazine N2H4 is not 2 ICl2- is linear but NH2- is bent 3 Of the compounds mercuryII cyanate HgOCN2 and mercuryII fulminate HgCNO2 one is highly explosive the other is. ACCl4 BC2Cl4 CC3H6 DC3H8. So as we have understood the meaning of the term co-plane ie.
Trigonal Planar in shape. Well adopt these measures to resolve this issueStep One. Comments 0 Answer Explanation.
The correct answer is A BF3. For instance we conventionally say that H2C CCl2 11-dichloroethylene lays on the yz -plane and that its 2px atomic orbitals form the CC π bond since the principal C2 rotation axis is along the CC. Any molecule that contains a tetrasubstituted atom or a trisubstituted atom that is not in the thirteenth or fourteenth group or nitrogen cannot be entirely planar.
A little shorter than the average CC single bond length. Reply 1 0 0 Rich Text Editor ansReply112201. 52 The molecular structure of a molecule will differ from its electron-pair geometry if.
NCERT P Bahadur IIT-JEE Previous Year Narendra Awasthi MS Chauhan. Which of the following molecules isare not planar. Determine the hybridization of carbon atoms.
The A-B bonds and the A-C bonds have dipole moments of 43 D. M- Number of monovalent atoms linked to central atom. Thus dimethyl sulfoxide is not planar nor is cePH3 or methane.
Watch world class content mapped to each topic you want to learn for all your course reference books. Check Answer and Solution for above Chemistry question. Formamide is a planar molecule.
Explain the following. Which of the following is a planar molecule. Calculate the maximum amount of heat that can be produced from burning 100 mole of acetylene.
To find out when the molecule is planar or otherwise we have to draw a 3-dimensional diagram for that allene molecule. Chemistry questions and answers. Hint Co-planar molecule can be defined as the molecule in which all the atoms bonds lone pairs of electrons of the compound lie in the same planeCo-planar molecule suggests that it has taken two dimensional geometry.
Each element within the ring must have a p-orbital that is perpendicular to the ring hence the molecule is planar. Ethylene is a planar molecule whereas acetylene is a linear molecule. A planar molecule is a way to describe a molecule that lays flat on a particular plane.
Solved by verified expert. Sp 3 hybridized state. H X 2 C C C H X 2.
All the atoms of the molecules in the same plane two. Chemistry Science Organic chemistry BIOMEDICAL 203. Butadiene C4H6 is a planar molecule that has the following carboncarbon bond lengthsa Predict the bond angles around each of the carbon atoms and sketch the molecule.
NCERT NCERT Exemplar NCERT Fingertips Errorless Vol-1 Errorless Vol-2. Which of the following molecule is planar. 10 pts A trigonal planar molecule AB C has three polar bonds.
Start studying the CH 13 HW flashcards containing study terms like Use VSEPR theory to predict the electron-pair arrangement and the molecular geometry of ozone O3. BF3 has a triangular planar arrangement. Hence option C is the right answer.
H X 2 C C O. B From left to right what is the hybridization of each carbon atom in butadiene. The electron-pair arrangement is tetrahedral the molecular geometry is bent The electron-pair garrangement is trigonal planar.
N C H C C H C N. C Charge of cation. Among the many distinctive features of benzene its aromaticity is the major.
Trigonal Pyramid in shape. Which one of the following molecules is planar. Convert the following infrared wavelengths to cm-1a.
NCERT DC Pandey Sunil Batra HC Verma Pradeep Errorless. This means that the ring cannot contain a neutral sp 3 carbon. Editor toolbars Bold Keyboard shortcut CtrlB Italic Keyboard shortcut CtrlI InsertRemove Numbered List InsertRemove Bulleted List Link Keyboard shortcut CtrlL Unlink.
No the shape of XeF4 is square planar. But it is not working in this case. The central atom has at least one lone pair 52 A trigonal planar molecule will have bond angles of __________.
I had the idea that the compound in with central atom has s p hybridisation is planar or the compound in which all the atoms has the same hybridization. 1 Ethylene C2H4 is a planar molecule but hydrazine N2H4 is not 2 ICl2- is linear but NH2- is bent 3 Of the compounds mercuryII cyanate HgOCN2 and mercuryII fulminate HgCNO2 one is highly. Which molecule is planar.
Discuss the planarity from the molecule. Correct option is C H Number of orbitals involved in hybridization. A NF3 B NCl3 C PH3 D BF3.
PH3 NF3 and NCl3 have trigonal pyramidal geometry. Draw a diagram while using hybridized orbitals. Please type your answer before submitting.
Chemistry questions and answers. Please type your answer before submitting. Complete Step By Step Answer.
Ab question is which of the following is planar essay cl4nx 34 and therefore we are supposed to tell that which among the following it enough of that we would calculate aur find out the terrace number and their hybridization and from there we would find out there doctor and then we would find out that which one among this study number calculation we have a formula half of the.
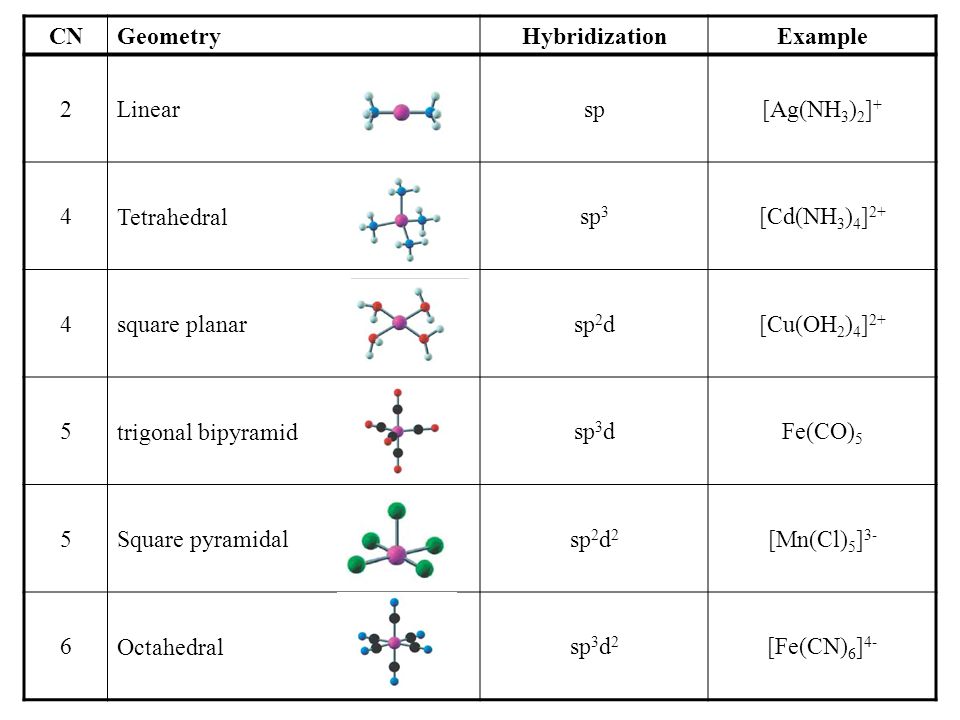
What Hybridization Is Generally Utilized By The Central Atom In A Square Planar Molecule Socratic

Different Possible Planar And Nonplanar Conformations For A Molecule Of Download Scientific Diagram

That Molecules With One And Two So 2 Groups Have A Planar Conformation Download Scientific Diagram
Comments
Post a Comment